Silver
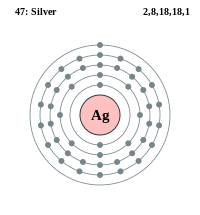
Silver (symbol Ag) ek chemical element hae jiske atomic number 47, aur atomic weight 107.86 a.m.u. hae. Iske symbol Ag, Latin me silver ke sabd , argentum se aais hae. Ii ek transition metal hae.
| Atomic Structure | |
|---|---|
| Symbol | Ag |
| Atomic Number | 47 |
| Atomic Mass | 107.86 g/mol |
| Periodic Table | |
| Group | |
| Row / Period | 5 |
| Element Category | Transition Metal |
| Chhapa | |
 | |
Properties
badloPhysical properties
badloSilver ek naram metal hae. Jab iske paisa nai to gahana me kaam me lawa jaae hae ab iske sona nai to aur koi metal ke saathe milawa jaawe hae jisse ki aur karraa hoe jaawe. Iske rang bluish-white hae, ii light ke achchhaa se reflect kare hae aur electricity ke achchhaa se conduct kare hae. Iske ek precious metal maana jaawe hae. Iske wire me banawa jaae sake hae aur ek sheet me hammer karaa jaawe sake hae. Dunis bhar me Silver ke paisa ke kharida aur beja jaae sake hae.
Chemical properties
badloSilver bahut eactive nai hae. Ii Nitric Acid ke chhorr ke aur acid me dissolve nai hoe hae. Lekin ii tagrraa oxidizing agent, jaise ki potassium dichromate nai to potassium permanganate se ract hoe hae. Iske bahut muskil se murcha pakrre hae. Ii khaali uu time murrchae hae jab ki ii hydrogen sulfide se hawaa me mile hae, jab ki ii ek karia coating fom kare hae.
Silver +1 oxidation state me ion ke ruup me rahe hae, compounds me jaise kisilver(I) oxide. Kuchh compound +2 oxidation state me rahe hae aur ii bahut tagrraa oxidizing agent rahe hae. Silver ke compound gray, black, brown, nai to ujjar rahe sake hae. Silver ke compund ke disinfectant ke rakam me kaam me lwa jaae sake hae.
Chhapa ke gallery
badlo-
A chunk of silver
-
Native silver on calcite
-
symbol for luna
-
symbol for luna
-
Křemičitan stříbrný - Ag2SiO3
-
Natural silver nugget
-
Silver foil in a bottle
Products
badloMiscellaneous
badloRoman and Byzantine
badlo
| Ii vigyan article ek chhota panna hae. Aap iske lamba karke Wikipedia ke madat kare saktaa hae. |